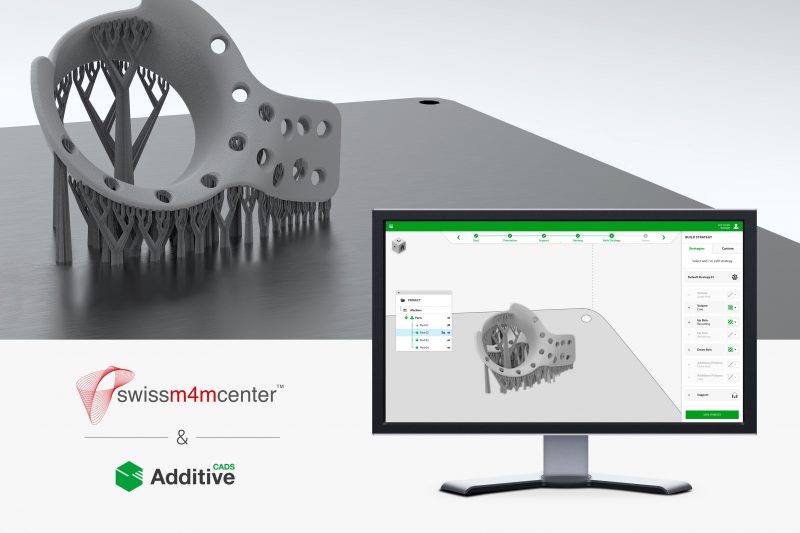
Swiss m4m Center relies on digital process chain of CADS Additive.
CADS Additive GmbH and the Swiss m4m Center, an additive manufacturing center for medical applications aiming for an ISO 13485 certified environment, are installing AM-Studio solutions for the digital mapping of the relevant manufacturing process chain in a PTC Creo® and PTC Windchill® environment.
The manufacturing of medical implants requires a multitude of preparatory and process steps. Starting with the acquisition of patient-specific data using imaging techniques, through the generation of implant geometries and their preparation for additive manufacturing using the Selective Laser Melting (SLM) process, up to the machining post-processing and final electroplating, it is necessary to manage a very large amount of data and map it as efficiently as possible. In addition to the quality management requirements defined in ISO 13485, the focus is primarily on establishing a digital twin of the implants to be manufactured. This twin is used in all design and manufacturing steps and thus forms the basis for an efficient and continuous process chain.
“CADS Additive allows us to map and control all necessary manufacturing steps and processes across manufacturers within the AM-Studio software family.”
Nicolas Bouduban, CEO, Swiss m4m Center AG
“As official development partner of PTC Inc. and specialist in data preparation and accompanying quality assurance of additive manufacturing processes, CADS Additive is the perfect partner for the implementation of the ambitious goals of the Swiss m4m Center,” Nicolas Bouduban, CEO of Swiss m4m, emphasizes. “CADS Additive allows us to map and control all necessary manufacturing steps and processes across manufacturers within the AM-Studio software family. The combination of the steps in one software tool reduces the effort from the user’s point of view tremendously,” Bouduban continues.
“The CADS group looks back on a history of more than 10 years in the field of software development for and with medical technology. The cooperation with the Swiss m4m Center is the highlight of this initiative in the field of combining the most diverse manufacturing processes and applications,” DI Peter Leitner, Head of Innovation of CADS Additive, explains. “The variety of manufacturing methods and machine manufacturers involved in this initiative poses a particular challenge, especially since all the components of this puzzle are interlocked and harmonized in an integrated solution. Our now 12 years of experience as a development partner for the products PTC Creo® and PTC Windchill® give us the necessary expertise and self-confidence to create a unique solution with and for Swiss m4m,” Leitner continues.
About Swiss m4m Center AG
The Swiss m4m Center is an additive manufacturing center for medical applications that promotes the development and use of 3D printing with a focus on process know-how in a validated environment. Together with our partner network, we establish a public access environment for the medical device industry. The goal is to understand the specific clinical needs, leverage 3D printing technology according to ISO 13485, and provide 3D printed products and services. www.swissm4m.ch